
Chemistry, 12.02.2020 04:42 gabrielbergemancat
Consider the reaction
H2(g) + 1 2 O2(g) → H2O(ℓ) + 286 kJ
How much H2 would have to be burned to yield America’s daily energy share of 260,000 kcal? (1 cal = 4.184 J)
1.) 1900 mol
2.) 3800 mol
3.) 7600 mol
4.) 1 mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
Consider the reaction
H2(g) + 1 2 O2(g) → H2O(ℓ) + 286 kJ
How much H2 would...
H2(g) + 1 2 O2(g) → H2O(ℓ) + 286 kJ
How much H2 would...
Questions



Mathematics, 16.10.2020 16:01

Social Studies, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01


Computers and Technology, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01

History, 16.10.2020 16:01



Social Studies, 16.10.2020 16:01


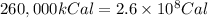 (Conversion factor: 1 kCal = 1000 Cal)
(Conversion factor: 1 kCal = 1000 Cal)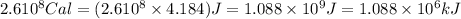
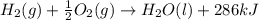
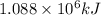 of energy will be released when
of energy will be released when 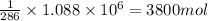 of hydrogen gas is consumed
of hydrogen gas is consumed

