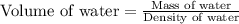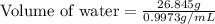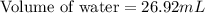Chemistry, 12.02.2020 04:41 carlosgc19
A student obtained a clean, dry, glass-stoppered flask. He weighed the flask stopper and found the total mass to be 32.634g. He then filled the flask with water, weighed again, and obtained a mass of 59.479g. At the temperature of the water, he found that its density was 0.9973 g/mL. a.) What was the mass of the water? (show work)b.) What was the volume of the water? (Show work)c.) What was the volume of the flask? (show work)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
A student obtained a clean, dry, glass-stoppered flask. He weighed the flask stopper and found the t...
Questions




English, 04.10.2019 21:30

Mathematics, 04.10.2019 21:30

Chemistry, 04.10.2019 21:30



Mathematics, 04.10.2019 21:30


Advanced Placement (AP), 04.10.2019 21:30



Biology, 04.10.2019 21:30

Biology, 04.10.2019 21:30


Biology, 04.10.2019 21:30

History, 04.10.2019 21:30