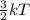A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. Sort the conditions based on the gas described. Some may have more than one answer, but noe will have the same answer.
1) Nitrogen has the greater value
2) Xenon has the greater value
3) Both gases have the same value
A. Gas that exerts the greater partial pressure.
B. Gas for which the molecules or atoms have the greatest average velocity
C. Gas that would have a higher rate of effusion through a small hole opened in the flask.
D. Gas for which the molecules or atoms have the greatest average of kinetic energy.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2

Chemistry, 23.06.2019 12:30
What are some examples of anthropogenic atmospheric particulates?
Answers: 1
You know the right answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. Sort th...
Questions



Chemistry, 26.10.2020 21:50


Mathematics, 26.10.2020 21:50

Mathematics, 26.10.2020 21:50

Mathematics, 26.10.2020 21:50

Mathematics, 26.10.2020 21:50


Geography, 26.10.2020 21:50

Health, 26.10.2020 21:50


Mathematics, 26.10.2020 21:50



Social Studies, 26.10.2020 21:50


History, 26.10.2020 21:50


Physics, 26.10.2020 21:50