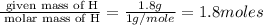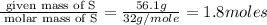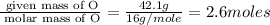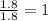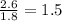A laboratory analysis of a 100 g sample finds it is composed of 1.8 g hydrogen, 56.1 g sulfur, and 42.1 g oxygen. What is its empirical formula? Give your answer in the form H#S#O#, where the number following the element’s symbol corresponds to the subscript in the formula. (Don’t include a 1 subscript explicitly.) For example, the formula CHO would be entered as CH2O.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1


You know the right answer?
A laboratory analysis of a 100 g sample finds it is composed of 1.8 g hydrogen, 56.1 g sulfur, and 4...
Questions

Physics, 29.11.2021 01:00

History, 29.11.2021 01:00



History, 29.11.2021 01:00










Mathematics, 29.11.2021 01:00

Mathematics, 29.11.2021 01:00