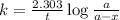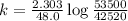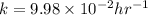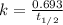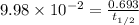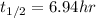Chemistry, 12.02.2020 04:58 harding6698
A sample of 28 Mg decays initially at a rate of 53500 disintegrations per minute, but the decay rate falls to 10980 disintegrations per minute after 48.0 hours
1.) What is the half life of 28 Mg in hours?
I have no idea how to work this equation. Please show step by step with correct answers so I can try to understand.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1

Chemistry, 23.06.2019 09:30
Where are the noble gases located in the periodic table? a. in the center b. on the left side c. in the upper right corner d. on the far right side
Answers: 1
You know the right answer?
A sample of 28 Mg decays initially at a rate of 53500 disintegrations per minute, but the decay rate...
Questions


History, 29.07.2019 23:00








Spanish, 29.07.2019 23:00


Chemistry, 29.07.2019 23:00

Mathematics, 29.07.2019 23:00



Physics, 29.07.2019 23:00

Biology, 29.07.2019 23:00