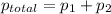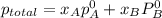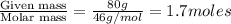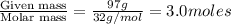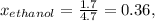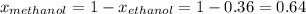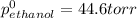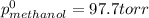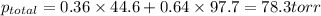Chemistry, 12.02.2020 05:28 usagimiller
At 293 K, methanol has a vapor pressure of 97.7 Torr and ethanol has a vapor pressure of 44.6 Torr. What would be the vapor pressure of a mixture of 80 g of ethanol and 97 g of methanol at 293 K?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

You know the right answer?
At 293 K, methanol has a vapor pressure of 97.7 Torr and ethanol has a vapor pressure of 44.6 Torr....
Questions

Mathematics, 30.12.2019 10:31


Mathematics, 30.12.2019 10:31

Mathematics, 30.12.2019 10:31

Mathematics, 30.12.2019 10:31

Physics, 30.12.2019 10:31


Mathematics, 30.12.2019 10:31

History, 30.12.2019 10:31

Mathematics, 30.12.2019 10:31



Physics, 30.12.2019 10:31

History, 30.12.2019 10:31

Mathematics, 30.12.2019 10:31

History, 30.12.2019 10:31


History, 30.12.2019 10:31

English, 30.12.2019 10:31

English, 30.12.2019 10:31

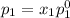 and
and 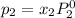
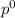 = pressure in the pure state
= pressure in the pure state