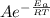Chemistry, 12.02.2020 05:30 dededese2403
Which of the following statements is TRUE?a. The rate constant does not depend on the activation energy for a reaction where the products are lower in energy than the reactants. b. The addition of a homogeneous catalyst does not change the activation energy of a given reaction. c. Rate constants are temperature dependent. d. None of the above is true. e. A catalyst raises the activation energy of a reaction.

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3
You know the right answer?
Which of the following statements is TRUE?a. The rate constant does not depend on the activation ene...
Questions



Mathematics, 03.02.2021 04:40

Mathematics, 03.02.2021 04:40

History, 03.02.2021 04:40



Mathematics, 03.02.2021 04:40

English, 03.02.2021 04:40



Social Studies, 03.02.2021 04:40

Mathematics, 03.02.2021 04:40

Mathematics, 03.02.2021 04:40

Mathematics, 03.02.2021 04:40

Mathematics, 03.02.2021 04:40

English, 03.02.2021 04:40



Biology, 03.02.2021 04:40