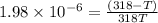Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

You know the right answer?
A certain liquid has a vapor pressure of 92.0 Torr at 23.0 ∘ C and 378.0 Torr at 45.0∘C.
A. C...
A. C...
Questions



History, 08.11.2019 08:31

Social Studies, 08.11.2019 08:31


Business, 08.11.2019 08:31







History, 08.11.2019 08:31

Social Studies, 08.11.2019 08:31


Biology, 08.11.2019 08:31




History, 08.11.2019 08:31

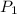 = 92.0 torr,
= 92.0 torr, 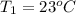 = (23 + 273)K = 296 K
= (23 + 273)K = 296 K
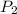 = 378.0 torr,
= 378.0 torr, 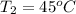 = (45 + 273)K = 318 K
= (45 + 273)K = 318 K
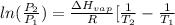
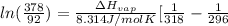
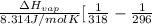
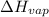 = 2926063.008 J/mol
= 2926063.008 J/mol
![ln (\frac{760 torr}{378 torr}) = -\frac{2926063.008 J/mol }{8.314 J/mol K} [\frac{1}{T} - \frac{1}{318}]](/tpl/images/0508/2864/327ff.png)