Chemistry, 12.02.2020 05:48 cristykianpour
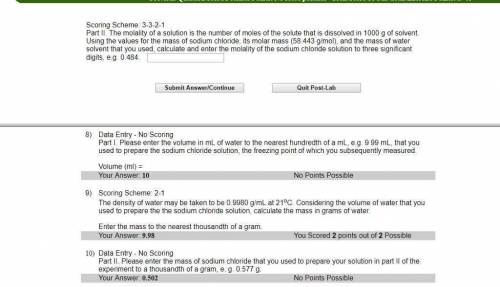
Part II. The molality of a solution is the number of moles of the solute that is dissolved in 1000 g of solvent. Using the values for the mass of sodium chloride, its molar mass (58.443 g/mol), and the mass of water solvent that you used, calculate and enter the molality of the sodium chloride solution to three significant digits, e. g. 0.484.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1


Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
Part II. The molality of a solution is the number of moles of the solute that is dissolved in 1000 g...
Questions









Physics, 10.03.2020 21:33




Chemistry, 10.03.2020 21:33






Mathematics, 10.03.2020 21:34

Mathematics, 10.03.2020 21:34