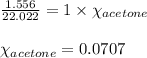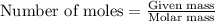Chemistry, 12.02.2020 05:58 mjlchance367
When a specific amount of acetone (C3H6O) is added to 100.0 g of pure water at 65°C, the vapor pressure of water over the solution is lowered by 1.556 kPa. Given the vapor pressure of water at 65°C is 25.022 kPa, what is the mass of acetone added?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

Chemistry, 23.06.2019 11:00
Just on number 2 (all parts), and if you do answer explain in detail
Answers: 3

You know the right answer?
When a specific amount of acetone (C3H6O) is added to 100.0 g of pure water at 65°C, the vapor press...
Questions


History, 15.04.2020 19:56

Computers and Technology, 15.04.2020 19:56






Mathematics, 15.04.2020 19:56


Mathematics, 15.04.2020 19:56









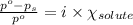
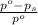 = relative lowering in vapor pressure = 1.556 kPa
= relative lowering in vapor pressure = 1.556 kPa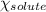 = mole fraction of solute = ?
= mole fraction of solute = ?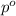 = vapor pressure of pure water = 22.022 kPa
= vapor pressure of pure water = 22.022 kPa