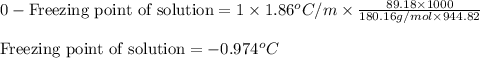Chemistry, 12.02.2020 18:31 gracebuffum
At 298 K, the osmotic pressure of a glucose solution (C6H12O6 (aq)) is 12.1 atm. Calculate the freezing point of the solution. The density of the solution is 1.034 g/mL.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
At 298 K, the osmotic pressure of a glucose solution (C6H12O6 (aq)) is 12.1 atm. Calculate the freez...
Questions

Mathematics, 22.04.2020 18:00


Biology, 22.04.2020 18:00


Biology, 22.04.2020 18:00

Mathematics, 22.04.2020 18:00


Mathematics, 22.04.2020 18:00




Biology, 22.04.2020 18:00

English, 22.04.2020 18:00


Mathematics, 22.04.2020 18:00


Mathematics, 22.04.2020 18:00

Mathematics, 22.04.2020 18:00

Mathematics, 22.04.2020 18:01

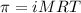
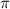 = osmotic pressure of the solution = 12.1 atm
= osmotic pressure of the solution = 12.1 atm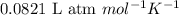
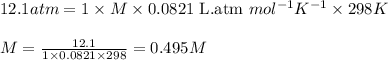
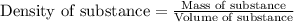
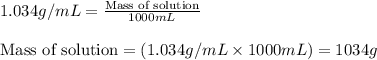
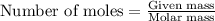
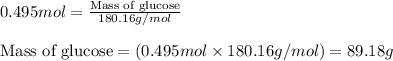
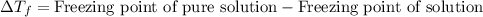
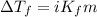
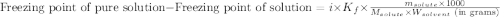
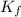 = molal freezing point elevation constant = 1.86°C/m
= molal freezing point elevation constant = 1.86°C/m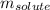 = Given mass of solute (glucose) = 89.18 g
= Given mass of solute (glucose) = 89.18 g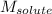 = Molar mass of solute (glucose) = 180.16 g/mol
= Molar mass of solute (glucose) = 180.16 g/mol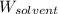 = Mass of solvent (water) = [1034 - 89.18] g = 944.82 g
= Mass of solvent (water) = [1034 - 89.18] g = 944.82 g