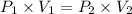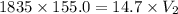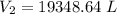Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3
You know the right answer?
A 155.0 −L helium tank contains pure helium at a pressure of 1835 psi and a temperature of 298 K. Ho...
Questions


Mathematics, 09.12.2020 18:20

Mathematics, 09.12.2020 18:20

Chemistry, 09.12.2020 18:20




Mathematics, 09.12.2020 18:20




Chemistry, 09.12.2020 18:20


Mathematics, 09.12.2020 18:20

Health, 09.12.2020 18:20

Social Studies, 09.12.2020 18:20

Mathematics, 09.12.2020 18:20

Computers and Technology, 09.12.2020 18:20