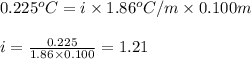Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
The freezing-point depression of a 0.100 m MgSO4 solution is 0.225°C. Determine the experimental van...
Questions


Mathematics, 20.10.2020 19:01

Mathematics, 20.10.2020 19:01

Geography, 20.10.2020 19:01

Mathematics, 20.10.2020 19:01






Social Studies, 20.10.2020 19:01

Business, 20.10.2020 19:01

Mathematics, 20.10.2020 19:01

History, 20.10.2020 19:01


Biology, 20.10.2020 19:01

Mathematics, 20.10.2020 19:01

Mathematics, 20.10.2020 19:01


Biology, 20.10.2020 19:01

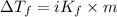
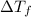 = depression in freezing point = 0.225°C
= depression in freezing point = 0.225°C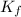 = Cryoscopic constant = 1.86°C/m
= Cryoscopic constant = 1.86°C/m