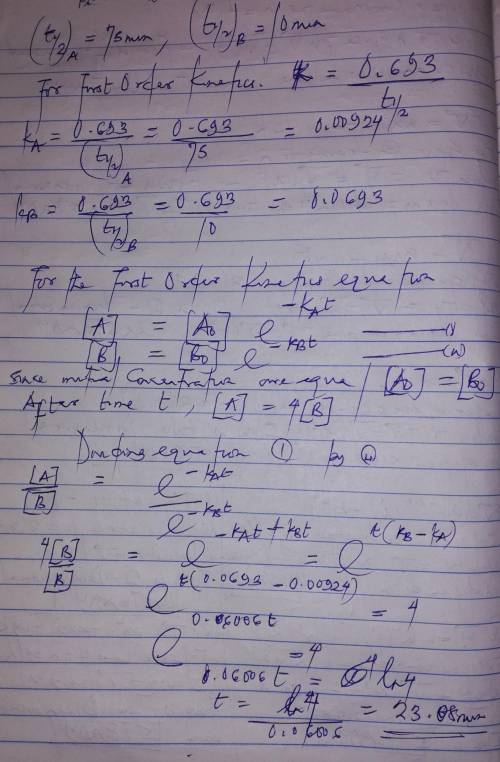Chemistry, 12.02.2020 22:50 sadieruegner393
A flask contains a mixture of compounds A and B. Both compounds decompose by first-order kinetics. The half-lives are 75.00 min for A and 10.00 min for B. If the concentrations of A and B are equal initially, how long will it take for the concentration of A to be four times that of B

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3
You know the right answer?
A flask contains a mixture of compounds A and B. Both compounds decompose by first-order kinetics. T...
Questions

Social Studies, 23.01.2020 17:31

English, 23.01.2020 17:31


History, 23.01.2020 17:31


Mathematics, 23.01.2020 17:31

Mathematics, 23.01.2020 17:31

English, 23.01.2020 17:31


Biology, 23.01.2020 17:31

Mathematics, 23.01.2020 17:31


Mathematics, 23.01.2020 17:31


Mathematics, 23.01.2020 17:31


History, 23.01.2020 17:31

Mathematics, 23.01.2020 17:31