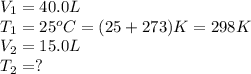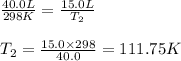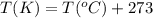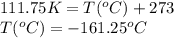Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Ethylene, C2H4, is used to make plastic milk bottles. When the volume of 1000 g of ethylene at 25oC...
Questions

Mathematics, 20.04.2020 21:27



Mathematics, 20.04.2020 21:27


History, 20.04.2020 21:27

Spanish, 20.04.2020 21:27






Biology, 20.04.2020 21:27


Mathematics, 20.04.2020 21:27



Arts, 20.04.2020 21:27


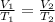
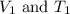 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.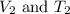 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.