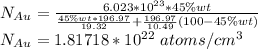Chemistry, 13.02.2020 00:38 seannalove4148
Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic centimeter for a silver-gold alloy that contains 45 wt% Au and 55 wt% Ag. The densities of pure gold and silver are 19.32 and 10.49 g/cm3, respectively. The atomic weight of Au is 196.97 g/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic c...
Questions

English, 24.02.2021 01:00


Spanish, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00



Mathematics, 24.02.2021 01:00




Mathematics, 24.02.2021 01:00

Medicine, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

Biology, 24.02.2021 01:00


Mathematics, 24.02.2021 01:00


Mathematics, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

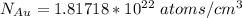
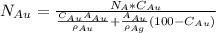
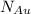 are number of gold atoms.
are number of gold atoms.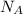 is Avogadro Number.
is Avogadro Number.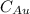 is the amount of gold.
is the amount of gold.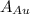 is the atomic weight of gold.
is the atomic weight of gold.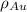 is the density of gold.
is the density of gold.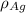 is the density of silver.
is the density of silver.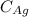 is the amount of silver.
is the amount of silver.