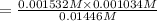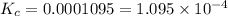Chemistry, 13.02.2020 03:48 emmaguentherp3hjd3
Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this: (aq)(l)(aq)(aq) At a certain temperature, a chemist finds that a reaction vessel containing an aqueous solution of hydrofluoric acid, water, fluoride anion, and hydronium cation at equilibrium has the following composition: compound amount Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this: (aq)(l)(aq...
Questions







English, 14.02.2020 23:56










Mathematics, 14.02.2020 23:58

Computers and Technology, 14.02.2020 23:58


History, 14.02.2020 23:58

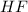 = 1.62 g
= 1.62 g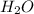 = 516 g
= 516 g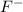 = 0.163 g
= 0.163 g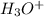 = 0.110 g
= 0.110 g .
.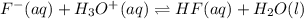
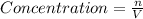
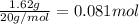
![[HF]=\frac{0.081 mol}{5.6 L}=0.01446 M](/tpl/images/0509/5531/82215.png)
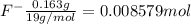
![[F^-]=\frac{0.008579 mol}{5.6 L}=0.001532 M](/tpl/images/0509/5531/21915.png)
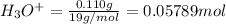
![[H_3O^+]=\frac{0.05789 mol}{5.6 L}=0.001034 M](/tpl/images/0509/5531/e5735.png)
![K_c=\frac{[F^-][H_3O^+]}{[HF]}](/tpl/images/0509/5531/d3418.png)