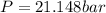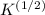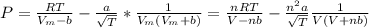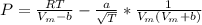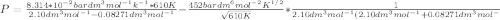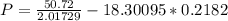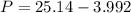Chemistry, 13.02.2020 04:53 Gearyjames8
Calculate the pressure exerted by benzene for a molar volume of 2.10 L at 610. K using the Redlich-Kwong equation of state: P==RTVm−b−aT√1Vm(Vm+b)nRTV−nb−n2aT√ 1V(V+nb) The Redlich-Kwong parameters a and b for benzene are 452.0 bar⋅dm6⋅mol−2⋅K1/2 and 0.08271 dm3⋅mol−1, respectively. Express your answer with the appropriate units.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

You know the right answer?
Calculate the pressure exerted by benzene for a molar volume of 2.10 L at 610. K using the Redlich-K...
Questions





Spanish, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

English, 16.10.2020 05:01

English, 16.10.2020 05:01


Social Studies, 16.10.2020 05:01


English, 16.10.2020 05:01

Geography, 16.10.2020 05:01

Spanish, 16.10.2020 05:01



Health, 16.10.2020 05:01


Geography, 16.10.2020 05:01