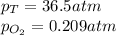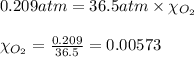Chemistry, 13.02.2020 05:14 jrassicworld4ever
What should be the mole fraction of O2 in the gas mixture the diver breathes in order to have the same partial pressure of oxygen in his lungs as he would at sea level? Note that the mole fraction of oxygen at sea level is 0.209.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1
You know the right answer?
What should be the mole fraction of O2 in the gas mixture the diver breathes in order to have the sa...
Questions



Physics, 16.04.2020 00:06


Mathematics, 16.04.2020 00:06








Computers and Technology, 16.04.2020 00:06


Mathematics, 16.04.2020 00:06

Mathematics, 16.04.2020 00:06

English, 16.04.2020 00:06


Mathematics, 16.04.2020 00:06

History, 16.04.2020 00:06

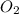 in the gas mixture the diver breathes in order to have the same partial pressure of oxygen in his lungs as he would at sea level? Note that the mole fraction of oxygen at sea level is 0.209.
in the gas mixture the diver breathes in order to have the same partial pressure of oxygen in his lungs as he would at sea level? Note that the mole fraction of oxygen at sea level is 0.209.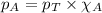 ........(1)
........(1)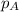 = partial pressure of oxygen at sea level = ?
= partial pressure of oxygen at sea level = ?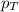 = total pressure at sea level = 1.00 atm
= total pressure at sea level = 1.00 atm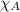 = mole fraction of oxygen at sea level = 0.209
= mole fraction of oxygen at sea level = 0.209