
Chemistry, 13.02.2020 05:55 jasonoliva13
Which of the following sets are isoelectronic (i. e., have the same number of electrons)? i. Br-, Kr, Sr2+ ii. C, N-, O2- iii. Mg2+, Ca2+, Sr2+ iv. O2-, O, O2+ v. Ag+, Cd2+, Pd

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
You know the right answer?
Which of the following sets are isoelectronic (i. e., have the same number of electrons)? i. Br-, Kr...
Questions



Mathematics, 25.07.2019 08:00

Mathematics, 25.07.2019 08:00


Mathematics, 25.07.2019 08:00

Computers and Technology, 25.07.2019 08:00











History, 25.07.2019 08:00

Mathematics, 25.07.2019 08:00

History, 25.07.2019 08:00

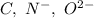
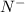 has 6 electrons.
has 6 electrons.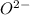 has 6 electrons.
has 6 electrons.

