Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

You know the right answer?
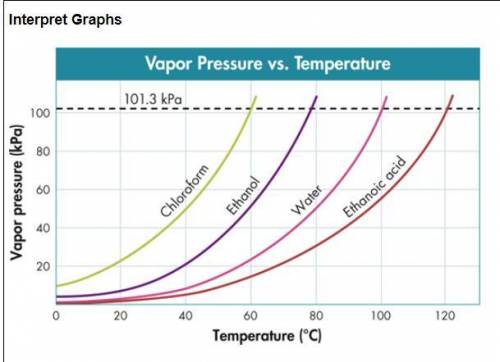
Use the figure below to determine to boiling point of -Chloroform at 80 kPa -Ethanol at 20 kPa -Etha...
Questions

History, 14.10.2019 06:30

History, 14.10.2019 06:30

Spanish, 14.10.2019 06:30


Health, 14.10.2019 06:30



Mathematics, 14.10.2019 06:30




Mathematics, 14.10.2019 06:30



Mathematics, 14.10.2019 06:30



History, 14.10.2019 06:30

History, 14.10.2019 06:30

Mathematics, 14.10.2019 06:30