
You considered the properties of two acid-base indicators, phenolphthalein and methyl orange. Many indicators are weak acids in water and establish the equilibrium: HIn(aq)(Color 1)+ H2O(l) → H3O (aq) + In-(aq)(Color 2). Indicators change color depending on whether they are in a protonated (HIn) or unprotonated (In-) form. What is the equilibrium expression for the phenolphthalein indicator in water and what colors are the protonated and unprotonated forms of the indicator?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
You considered the properties of two acid-base indicators, phenolphthalein and methyl orange. Many i...
Questions


Mathematics, 25.10.2020 19:30

Mathematics, 25.10.2020 19:30





Mathematics, 25.10.2020 19:30





Mathematics, 25.10.2020 19:30


Computers and Technology, 25.10.2020 19:30





Mathematics, 25.10.2020 19:30

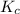
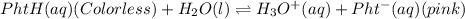
![K_c=\frac{[H_3O^+][Pht^-]}{[PhtH]}](/tpl/images/0509/9728/68136.png)


