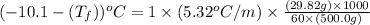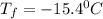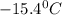A certain substance X has a normal freezing point of -10.1 degree C and a molal freezing point depression constant K_f = 5.32 degree C middot kg middot mol^-1. Calculate the freezing point of a solution made of 29.82 g of urea ((NH_2)_CO) dissolved in 500. g of X. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
A certain substance X has a normal freezing point of -10.1 degree C and a molal freezing point depre...
Questions

Mathematics, 20.09.2019 18:00


Health, 20.09.2019 18:00

Mathematics, 20.09.2019 18:00




Mathematics, 20.09.2019 18:00


Social Studies, 20.09.2019 18:00

Social Studies, 20.09.2019 18:00


Mathematics, 20.09.2019 18:00

Social Studies, 20.09.2019 18:00

Mathematics, 20.09.2019 18:00

Biology, 20.09.2019 18:00

History, 20.09.2019 18:00

Business, 20.09.2019 18:00

History, 20.09.2019 18:00

Mathematics, 20.09.2019 18:00

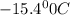
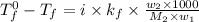
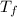 = boiling point of solution = ?
= boiling point of solution = ?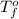 = boiling point of solvent (X) =
= boiling point of solvent (X) = 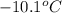
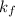 = freezing point constant =
= freezing point constant = 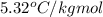
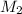 = molar mass of solute (urea) = 60 g/mol
= molar mass of solute (urea) = 60 g/mol