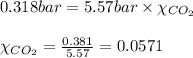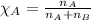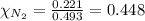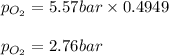Chemistry, 13.02.2020 18:57 AJSkullcrusher
A student has a 2.19 L bottle that contains a mixture of O 2 , N 2 , and CO 2 with a total pressure of 5.57 bar at 298 K . She knows that the mixture contains 0.221 mol N 2 and that the partial pressure of CO 2 is 0.318 bar . Calculate the partial pressure of O 2.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 22.06.2019 23:00
Need asap question 1 minerals are organic compounds. true false question 2 what vitamin can be found in foods like oranges, grapefruits, and broccoli? a. vitamin a b. vitamin k c.vitamin c d. vitamin d question 3 what are minerals? a. chemical elements that are needed for body processes. b. organic compounds that the body needs in small amounts to function properly. c. small molecules used to build proteins. d. an organic compound that is insoluble in water and includes fats. question 4 how many types of vitamins does the human body need? a. 15 b. 11 c. 13 d. 17 question 5 vitamins are a good source of energy. true false
Answers: 1
You know the right answer?
A student has a 2.19 L bottle that contains a mixture of O 2 , N 2 , and CO 2 with a total pressure...
Questions

Mathematics, 28.11.2019 05:31











Computers and Technology, 28.11.2019 05:31

Mathematics, 28.11.2019 05:31







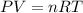
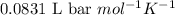
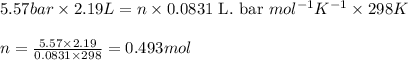
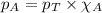 ........(1)
........(1)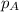 = partial pressure of carbon dioxide = 0.318 bar
= partial pressure of carbon dioxide = 0.318 bar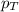 = total pressure = 5.57 bar
= total pressure = 5.57 bar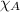 = mole fraction of carbon dioxide = ?
= mole fraction of carbon dioxide = ?