
Chemistry, 13.02.2020 18:57 jamilamiller200
When an antacid tablet dissolves in water, the fizz is due to a reaction between sodium hydrogen carbonate (sodium bicarbonate, NaHCO3) and citric acid (H3C6H5O7). 3 NaHCO3(aq) H3C6H5O7(aq) 3 CO2(g) 3 H2O(l) Na3C6H5O7(aq) How many moles of Na3C6H5O7 can be produced if one tablet containing 0.0370 mol of NaHCO3 is dissolved

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1
You know the right answer?
When an antacid tablet dissolves in water, the fizz is due to a reaction between sodium hydrogen car...
Questions

History, 14.04.2021 22:00



Mathematics, 14.04.2021 22:00





Mathematics, 14.04.2021 22:00


Biology, 14.04.2021 22:00


Spanish, 14.04.2021 22:00

Business, 14.04.2021 22:00


Mathematics, 14.04.2021 22:00

History, 14.04.2021 22:00

Mathematics, 14.04.2021 22:00


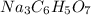 produced can be, 0.0123 mole
produced can be, 0.0123 mole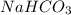 = 0.0370 mol
= 0.0370 mol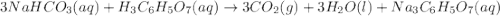
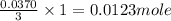 of
of 

