
Chemistry, 13.02.2020 20:02 connorgking
A. Based on the activation energies and frequency factors, rank the following reactions from fastest to slowest reaction rate, assuming they are all at the same temperature and that each starts with the same initial concentration.
E, 50 kJ/mol E,-350 kJ/mol 50 kJ/mol
A = 1.5 × 10-7 s-i A = 1.9 × 10-7 s-i A = 1.5 × 10-7 s-1
Fraction of molecules
The exponential term in the Arrhenius equation is equal to the fraction of molecules, f, with kinetic energy greater than or equal to the activation energy: f=e?Ea/(R?T). Most scientific calculators have an exfunction as the second function of the LN button.
B. A certain reaction with an activation energy of 165 kJ/mol was run at 505 K and again at 525 K . What is the ratio of f at the higher temperature to f at the lower temperature?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1


Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
A. Based on the activation energies and frequency factors, rank the following reactions from fastest...
Questions

Mathematics, 26.07.2019 15:30

Business, 26.07.2019 15:30

History, 26.07.2019 15:30


History, 26.07.2019 15:30



Mathematics, 26.07.2019 15:30

History, 26.07.2019 15:30











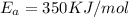 ,
, 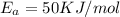 ,
, 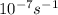 , A = 1.9×
, A = 1.9×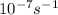 , A=1.5×
, A=1.5×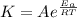
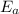 = Activation Energy
= Activation Energy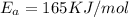
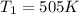
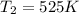
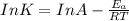
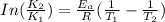
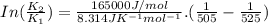
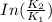 = 19846.04×7.544×
= 19846.04×7.544×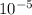 = 1.497
= 1.497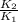 =
=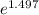 = 4.469
= 4.469 

