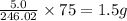Chemistry, 13.02.2020 20:54 golderhadashaowtatz
The mineral orpiment, having the empirical formula As2S3, was used in ancient times as a cosmetic. What mass of arsenic is present in 5.0 g of orpiment? Hint: Determine the percent composition of As in As2S3 , then take that percent of 5.0 g.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
You know the right answer?
The mineral orpiment, having the empirical formula As2S3, was used in ancient times as a cosmetic. W...
Questions


History, 17.01.2020 02:31


Chemistry, 17.01.2020 02:31



Biology, 17.01.2020 02:31


Physics, 17.01.2020 02:31

Mathematics, 17.01.2020 02:31

Chemistry, 17.01.2020 02:31

History, 17.01.2020 02:31


Mathematics, 17.01.2020 02:31



English, 17.01.2020 02:31

Mathematics, 17.01.2020 02:31


Mathematics, 17.01.2020 02:31

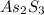 is, 246.02 g/mol and the molar mass of As is, 75 g/mol.
is, 246.02 g/mol and the molar mass of As is, 75 g/mol.