
Chemistry, 13.02.2020 20:57 mapoohdoll
Initially, a closed flask contains a mixture of N2 at a partial pressure of 3 atm and H2 at a partial pressure of 5 atm. The mixture reaches equilibrium and the partial pressure of NH3 is 2 atm. What is the value of the equilibrium constant?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Aforce of attraction/repulsion due to the spin of electrons what is this force?
Answers: 2

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
Initially, a closed flask contains a mixture of N2 at a partial pressure of 3 atm and H2 at a partia...
Questions


Mathematics, 22.04.2020 15:35







History, 22.04.2020 15:36



Biology, 22.04.2020 15:36


Chemistry, 22.04.2020 15:36

English, 22.04.2020 15:36

Mathematics, 22.04.2020 15:37

Mathematics, 22.04.2020 15:37

Mathematics, 22.04.2020 15:37

Mathematics, 22.04.2020 15:37

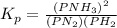 Hence we have
Hence we have

