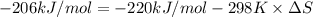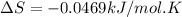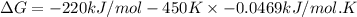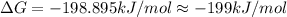For the gaseous reaction of carbon monoxide and chlorine to form phosgene (COCL2)
perform the following calculations
A) calculate delta S at 298k ( delta H= -220.kj/mol and delta G= -206kj/mol
B) assuming that delta S and delta H change little with temperature, calculate at 450k

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

You know the right answer?
For the gaseous reaction of carbon monoxide and chlorine to form phosgene (COCL2)
perfor...
perfor...
Questions



English, 04.11.2020 19:50


Mathematics, 04.11.2020 19:50

Mathematics, 04.11.2020 19:50

Mathematics, 04.11.2020 19:50

Physics, 04.11.2020 19:50

Computers and Technology, 04.11.2020 19:50

Computers and Technology, 04.11.2020 19:50


English, 04.11.2020 19:50

Mathematics, 04.11.2020 19:50




Advanced Placement (AP), 04.11.2020 19:50



History, 04.11.2020 19:50

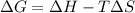
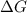 = Gibbs free energy = -206 kJ/mol
= Gibbs free energy = -206 kJ/mol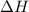 = enthalpy change = -220 kJ/mol
= enthalpy change = -220 kJ/mol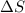 = entropy change = ?
= entropy change = ?