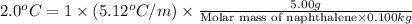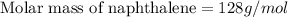Chemistry, 13.02.2020 21:27 jnsoccerboy3121
Calculate the molar mass of naphthalene, the organic compound in mothballs, if a solution prepared by dissolving 5.00 g of naphthalene in exactly 100 g of benzene has a freezing point 2.0°C below that of pure benzene. (Kf of benzene is 5.12°C/m.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
You know the right answer?
Calculate the molar mass of naphthalene, the organic compound in mothballs, if a solution prepared b...
Questions



Mathematics, 04.03.2020 09:36



History, 04.03.2020 09:37


Mathematics, 04.03.2020 09:37

Mathematics, 04.03.2020 09:38

Mathematics, 04.03.2020 09:38


Computers and Technology, 04.03.2020 09:39

Mathematics, 04.03.2020 09:39


Physics, 04.03.2020 09:39

Mathematics, 04.03.2020 09:39




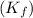 for benzene =
for benzene = 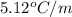
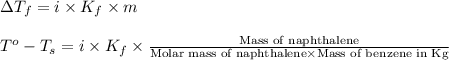
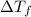 = change in freezing point =
= change in freezing point = 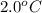
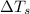 = freezing point of solution
= freezing point of solution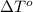 = freezing point of benzene
= freezing point of benzene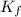 = freezing point constant for benzene =
= freezing point constant for benzene =