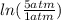Chemistry, 13.02.2020 22:22 haileyrae187
One mole of an ideal gas is contained in a cylinder with a movable piston. The temperature is constant at 778C. Weights are removed suddenly from the piston to give the following sequence of three pressures: a. P1 5 5.00 atm (initial state) b. P2 5 2.24 atm c. P3 5 1.00 atm (final state)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
One significant difference between an ionic bond, where electrons are taken from one atom and added to another atom, and a covalent or metallic bond, where electrons are shared is
Answers: 2

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
One mole of an ideal gas is contained in a cylinder with a movable piston. The temperature is consta...
Questions

Mathematics, 21.01.2020 19:31


Mathematics, 21.01.2020 19:31


Biology, 21.01.2020 19:31



Health, 21.01.2020 19:31





Physics, 21.01.2020 19:31

Mathematics, 21.01.2020 19:31


Biology, 21.01.2020 19:31




Spanish, 21.01.2020 19:31

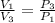
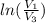 =
= 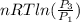
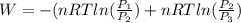 or
or 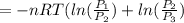
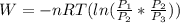 =
= 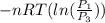 therefore the equation is the same for calculating directly from the initial pressure P₁, to the final pressure P₃
therefore the equation is the same for calculating directly from the initial pressure P₁, to the final pressure P₃