
A 3.76 g sample of a compound consisting of carbon, hydrogen, oxygen, nitrogen, and sulfur was combusted in excess oxygen. This produced 4.06 g CO 2 and 2.22 g H 2 O . A second sample of this compound with a mass of 5.69 g produced 3.73 g SO 3 . A third sample of this compound with a mass of 8.53 g produced 4.40 g HNO 3 . Determine the empirical formula of the compound. Enter the correct subscripts on the given chemical formula.?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1
You know the right answer?
A 3.76 g sample of a compound consisting of carbon, hydrogen, oxygen, nitrogen, and sulfur was combu...
Questions

Mathematics, 02.12.2020 06:00


Arts, 02.12.2020 06:00


Mathematics, 02.12.2020 06:00


Biology, 02.12.2020 06:00


Mathematics, 02.12.2020 06:00


Mathematics, 02.12.2020 06:00


History, 02.12.2020 06:00


Mathematics, 02.12.2020 06:00



History, 02.12.2020 06:00

History, 02.12.2020 06:00

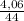 = 0,092g/mol
= 0,092g/mol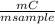 . 100 =
. 100 = 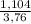 . 100 = 29,4%
. 100 = 29,4%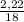 = 0,124 g/mol
= 0,124 g/mol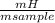 . 100 =
. 100 = 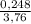 . 100 = 6,6%
. 100 = 6,6%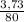 = 0,046g/mol
= 0,046g/mol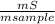 . 100 =
. 100 = 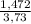 . 100 = 39,5%
. 100 = 39,5%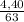 = 0,07
= 0,07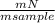 . 100 =
. 100 = 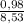 . 100 = 11,5%
. 100 = 11,5%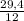 = 2,45 mol
= 2,45 mol

