
Chemistry, 29.09.2019 07:10 genyjoannerubiera
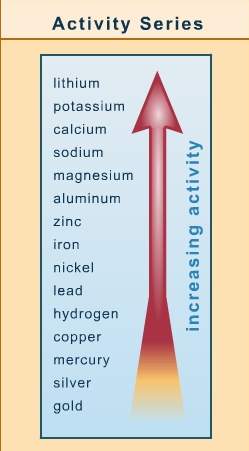
Refer to the activity series chart and determine which of the following displacement reactions can occur.
a. 2ag(s) + zn(no3)2(aq) → 2agno3(aq) + zn(s)
b. 2cu(no3)2(aq) + 2ag(s) → 2agno3(aq) + cu(s)
c. cuso4(aq) + fe(s) → feso4(aq) + cu(s)
d. 3mgso4(aq) + 2al(s) → al2(so4)3(aq) + 3mg(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
Refer to the activity series chart and determine which of the following displacement reactions can o...
Questions


Mathematics, 23.10.2019 08:00

History, 23.10.2019 08:00




Health, 23.10.2019 08:00


Biology, 23.10.2019 08:00

History, 23.10.2019 08:00


English, 23.10.2019 08:00

Advanced Placement (AP), 23.10.2019 08:00


English, 23.10.2019 08:00







