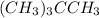Chemistry, 14.02.2020 01:44 finlchey3860
Place the following compounds in order of decreasing strength of intermolecular forces. I. CH3CH2CH2CH2CH2CH3 II. (CH3)3CCH3 III. (CH3)3CCH2CH3 Group of answer choices

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
You know the right answer?
Place the following compounds in order of decreasing strength of intermolecular forces. I. CH3CH2CH2...
Questions

History, 11.05.2021 09:20

Mathematics, 11.05.2021 09:20

Mathematics, 11.05.2021 09:20




Mathematics, 11.05.2021 09:20

Mathematics, 11.05.2021 09:20

History, 11.05.2021 09:20

Geography, 11.05.2021 09:20


Computers and Technology, 11.05.2021 09:20


Mathematics, 11.05.2021 09:20

Chemistry, 11.05.2021 09:20

Social Studies, 11.05.2021 09:20




Mathematics, 11.05.2021 09:20

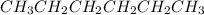 >
> 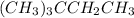 >
>