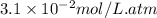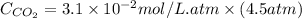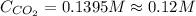Chemistry, 14.02.2020 02:27 cookie42087
Calculate the concentration of CO2 in water at 25 degrees Celsius when the pressure of CO2 over the solution is 4.5 atm. At 25 degrees Celsius, the Henry's law constant for CO2 in water is 3.1 * 10^-2 mol/L*atm.
? M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
Calculate the concentration of CO2 in water at 25 degrees Celsius when the pressure of CO2 over the...
Questions


Mathematics, 10.09.2019 01:20



Mathematics, 10.09.2019 01:20



Mathematics, 10.09.2019 01:20

Mathematics, 10.09.2019 01:20

Mathematics, 10.09.2019 01:20

Mathematics, 10.09.2019 01:20

History, 10.09.2019 01:20



Mathematics, 10.09.2019 01:30

Mathematics, 10.09.2019 01:30


Computers and Technology, 10.09.2019 01:30


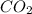 is, 0.12 M
is, 0.12 M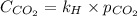
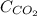 = concentration of
= concentration of 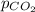 = partial pressure of
= partial pressure of 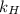 = Henry's law constant =
= Henry's law constant =