Chemistry, 14.02.2020 02:48 seiglersteven99
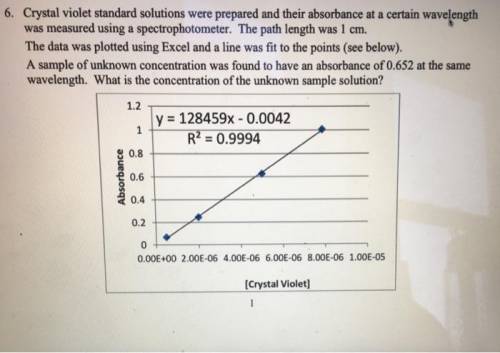
Crystal violet standard solutions were prepared and their absorbance at a certain wavelength was measured using a spectrophotometer. The path length was 1 cm. The data was plotted using Excel and a line was fit to the points (see below). A sample of unknown concentration was found to have an absorbance of 0.652 at the same wavelength. (a) What is the molar absorptivity of this compound at the certain wavelength? (b) What is the concentration of the unknown sample solution?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1


Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3
You know the right answer?
Crystal violet standard solutions were prepared and their absorbance at a certain wavelength was mea...
Questions


Mathematics, 05.10.2021 02:50

History, 05.10.2021 02:50



English, 05.10.2021 02:50

Mathematics, 05.10.2021 02:50

SAT, 05.10.2021 02:50




Mathematics, 05.10.2021 02:50

Mathematics, 05.10.2021 02:50

Mathematics, 05.10.2021 02:50



English, 05.10.2021 02:50


Physics, 05.10.2021 02:50

History, 05.10.2021 02:50