
Chemistry, 14.02.2020 02:44 SKSKSKSKGKUFHjk
Write the equilibrium-constant expression for the reactionA(s)+3B(l)↽−−⇀2C(aq)+D(aq)A (s)+3B(l)↽−−⇀2C(aq)+D(aq)in terms of [A], [B], [C], and [D] as needed. Note that KcKc, which is sometimes symbolized as KeqKeq, denotes that the equilibrium constant is expressed using molar concentrations. For this question, KcKc means the same thing as KeqKeq. Kc=.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2


Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1
You know the right answer?
Write the equilibrium-constant expression for the reactionA(s)+3B(l)↽−−⇀2C(aq)+D(aq)A (s)+3B(l)↽−−⇀2...
Questions



English, 28.01.2021 07:00

Mathematics, 28.01.2021 07:00

Mathematics, 28.01.2021 07:00

Mathematics, 28.01.2021 07:00





Computers and Technology, 28.01.2021 07:00


French, 28.01.2021 07:00




Arts, 28.01.2021 07:00

English, 28.01.2021 07:00

Mathematics, 28.01.2021 07:00

![K_{c} = [\text{C}]^{2}[\text{[D]}](/tpl/images/0511/0111/07ffc.png)
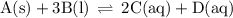
![K_{c} = \dfrac{[\text{Products}]}{[\text{Reactants}]}](/tpl/images/0511/0111/68d06.png)
![K_{c} = [\textbf{C}]^{\mathbf{2}}\textbf{[D]}](/tpl/images/0511/0111/3e70c.png)


