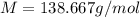An element, X, can form a chloride (XCl3) and an iodide (XI3). The chloride can be converted quantitatively into the iodide when heated and exposed to excess iodine. If 0.760 grams of XCl3 are heated with iodine, 1.610 g of XI3 are produced. What is the chemical symbol for this element? Use stoichiometry to create an equation that allows you to solve for the molecular weight of X.2 XCl3 + 3 I2 > 2 XI3 + 3 Cl2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1


Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
An element, X, can form a chloride (XCl3) and an iodide (XI3). The chloride can be converted quantit...
Questions

Mathematics, 07.11.2019 05:31

Social Studies, 07.11.2019 05:31



English, 07.11.2019 05:31

Mathematics, 07.11.2019 05:31






Geography, 07.11.2019 05:31



History, 07.11.2019 05:31

Biology, 07.11.2019 05:31

Biology, 07.11.2019 05:31



Chemistry, 07.11.2019 05:31

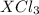 = 0.760 g
= 0.760 g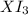 = 1.610 g
= 1.610 g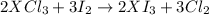
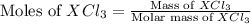
![\text{Moles of }XCl_3=\frac{0.760}{[M+3(35.5)]}](/tpl/images/0511/1010/d1e30.png)
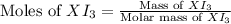
![\text{Moles of }XI_3=\frac{1.610}{[M+3(126.9)]}](/tpl/images/0511/1010/a64e9.png)
![\frac{0.760}{[M+3(35.5)]}=\frac{1.610}{[M+3(126.9)]}](/tpl/images/0511/1010/56351.png)
![\frac{0.760}{[M+106.5]}=\frac{1.610}{[M+380.7]}](/tpl/images/0511/1010/dbce0.png)