

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
A rock is believed to have been formed 1.25 billion years ago, as calculated by using potassium-40 d...
Questions

Social Studies, 17.10.2019 17:30

English, 17.10.2019 17:30

History, 17.10.2019 17:30

Mathematics, 17.10.2019 17:30

History, 17.10.2019 17:30





Chemistry, 17.10.2019 17:30




Chemistry, 17.10.2019 17:30

Mathematics, 17.10.2019 17:30

Physics, 17.10.2019 17:30




Mathematics, 17.10.2019 17:30

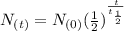
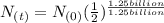 =
= 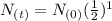 =0.5 of
=0.5 of 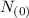 will be left or 50 % of the original amount of potassium 40 will be left
will be left or 50 % of the original amount of potassium 40 will be left

