
You double the partial pressure of a gas over a liquid at constant temperature. Which of these statements is then true? a. The Henry’s Law constant is decreased by half. b. There are half as many gas molecules in the liquid. c. There is no change in the number of gas molecules in the liquid. d. The Henry’s Law constant is doubled. e. There are twice as many gas molecules in the liquid.

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 13:30
These traits describe either a chemical or a nuclear reaction. which statements describe a nuclear reaction? check all that apply. involves the loss, gain, or sharing of electrons may involve a change in total mass involve relatively low energy changes occur outside the nucleus involve very high-energy changes involve changes in nuclides when decay occurs
Answers: 1

Chemistry, 23.06.2019 16:00
Which part of the mantle is similar to the crust ? (science earth's layers)
Answers: 1

Chemistry, 23.06.2019 19:30
Is the following chemical equation balanced? agno3 + nacl 4agcl + nano3 yes no
Answers: 1
You know the right answer?
You double the partial pressure of a gas over a liquid at constant temperature. Which of these state...
Questions

Mathematics, 05.10.2021 01:00



Mathematics, 05.10.2021 01:00






Mathematics, 05.10.2021 01:00

Mathematics, 05.10.2021 01:00

Business, 05.10.2021 01:00





History, 05.10.2021 01:00



Mathematics, 05.10.2021 01:00

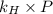
 = Henry's constant
= Henry's constant

