
Reduction and oxidation must occur together. The electrons from the oxidized species are transferred to the reduced species. Chemists often break these two processes apart and write what are referred to as "half reactions." Consider these two half reactions: Zn2+ (aq) + 2 e- → Zn (s) Cu2+ (aq) + 2 e- à Cu (s) a. Are these oxidation or reduction half reactions? b. Calculate ΔG° for each of these reactions. Note that ΔG°f for the electron is 0 kJ/mol. c. Based on your answer to (b), does Zn2+ or Cu2+ more strongly favor reduction?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
Reduction and oxidation must occur together. The electrons from the oxidized species are transferred...
Questions

Mathematics, 01.12.2019 20:31

Social Studies, 01.12.2019 20:31

Arts, 01.12.2019 20:31


English, 01.12.2019 20:31



History, 01.12.2019 20:31





History, 01.12.2019 20:31

English, 01.12.2019 20:31


Biology, 01.12.2019 20:31

Chemistry, 01.12.2019 20:31

Social Studies, 01.12.2019 20:31


Chemistry, 01.12.2019 20:31

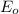 are as follows.
are as follows.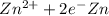 ,
, 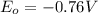
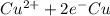 ,
, 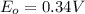
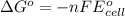
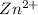 is as follows.
is as follows.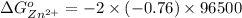
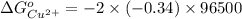
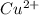 is the best oxidizing agent.
is the best oxidizing agent.

