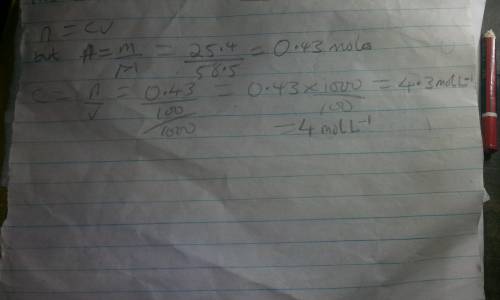Chemistry, 14.02.2020 05:26 zackarygonzalez1028
A chemist prepares a solution of sodium chloride(NaCl) by measuring out 25.4g of sodium chloride into a 100ml volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's sodium chloride solution. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
A chemist prepares a solution of sodium chloride(NaCl) by measuring out 25.4g of sodium chloride int...
Questions

Computers and Technology, 27.08.2019 14:30




Biology, 27.08.2019 14:30


Mathematics, 27.08.2019 14:30

Chemistry, 27.08.2019 14:30


Mathematics, 27.08.2019 14:30

Biology, 27.08.2019 14:30




English, 27.08.2019 14:30

Social Studies, 27.08.2019 14:30



English, 27.08.2019 14:30

English, 27.08.2019 14:30