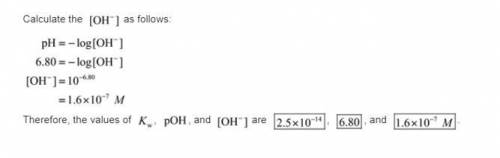. Like any equilibrium constant, Kw, changes with temperature. (a) Given that autoionization is endothermic, how does Kw change with rising T? Explain with a reaction that includes heat as a reactant or product. (b) In many medical applications, the value of Kw at 37°C (body T) may be more appropriate than the value at 25°C, 1.0x10-14. The pH of pure at 37°C is 6.80. Calculate Kw, pOH and [OH-] at this T.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3


Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
. Like any equilibrium constant, Kw, changes with temperature. (a) Given that autoionization is endo...
Questions




History, 14.01.2021 04:20

Geography, 14.01.2021 04:20

Chemistry, 14.01.2021 04:20



Mathematics, 14.01.2021 04:20




French, 14.01.2021 04:20


Mathematics, 14.01.2021 04:20

Mathematics, 14.01.2021 04:20

Chemistry, 14.01.2021 04:20



Mathematics, 14.01.2021 04:20

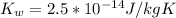
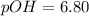
![[OH^{-}] =1.6*10^{-7} M](/tpl/images/0511/5212/a2f6d.png)