Chemistry, 14.02.2020 17:27 moldybubblegum11
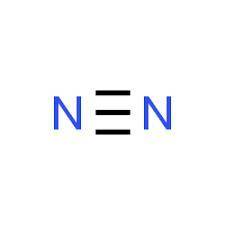
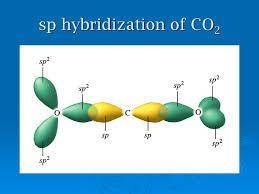
Which of the following compounds would have a linear molecular geometry? 1. N2 2. H2S 3. CO2 a. 1 and 3 only b.1 and 2 only c.2 and 3 only d.1,2 and 3 neither 1, 2,or 3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
Which of the following compounds would have a linear molecular geometry? 1. N2 2. H2S 3. CO2 a. 1 an...
Questions

Biology, 14.11.2020 03:10

Mathematics, 14.11.2020 03:10

Physics, 14.11.2020 03:10

Health, 14.11.2020 03:10

Mathematics, 14.11.2020 03:10

Mathematics, 14.11.2020 03:10


Physics, 14.11.2020 03:10

Mathematics, 14.11.2020 03:10

Mathematics, 14.11.2020 03:10

Social Studies, 14.11.2020 03:10







Business, 14.11.2020 03:10

Mathematics, 14.11.2020 03:10

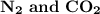 are the following compounds that have a linear molecular geometry. Option A is correct.
are the following compounds that have a linear molecular geometry. Option A is correct.