
Chemistry, 14.02.2020 17:26 danidavis2002
You have 50 ml of a complex mixture of weak acids that contains some HF (pKa = 3.18) and some HCN (pKa = 9.21). Which is larger, [F-]/[HF] or [CN-]/[HCN]?

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
You have 50 ml of a complex mixture of weak acids that contains some HF (pKa = 3.18) and some HCN (p...
Questions





Mathematics, 27.03.2021 02:10




Mathematics, 27.03.2021 02:10

Biology, 27.03.2021 02:10

Mathematics, 27.03.2021 02:10

Geography, 27.03.2021 02:10


Mathematics, 27.03.2021 02:10




English, 27.03.2021 02:10

English, 27.03.2021 02:10

![\frac{[F^{-}]}{[HF]}](/tpl/images/0511/5531/fe465.png) is larger
is larger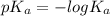 , where
, where 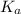 is the acid dissociation constant.
is the acid dissociation constant.![K_{a}=\frac{[H^{+}][A^{-}]}{[HA]}](/tpl/images/0511/5531/c814f.png) and
and ![\frac{[A^{-}]}{[HA]}=\frac{K_{a}}{[H^{+}]}](/tpl/images/0511/5531/b8841.png)
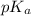
![[H^{+}]](/tpl/images/0511/5531/85507.png) will be constant.
will be constant.![(\frac{F^{-}}{[HF]}=\frac{K_{a}(HF)}{[H^{+}]})(\frac{CN^{-}}{[HCN]}=\frac{K_{a}(HCN)}{[H^{+}]})](/tpl/images/0511/5531/19b23.png)


