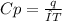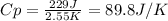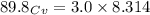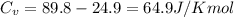Chemistry, 14.02.2020 17:25 postorivofarms
When 229 J of energy is supplied as heat to 3.0 mol of nitrogen N2(g) at constant pressure, the temperature of the sample increases by 2.55 K. Assuming that under these conditions nitrogen behaves as an ideal gas, what is the value of the molar heat capacity at constant volume of N2(g)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
When 229 J of energy is supplied as heat to 3.0 mol of nitrogen N2(g) at constant pressure, the temp...
Questions

Mathematics, 29.06.2021 22:10

English, 29.06.2021 22:10

Biology, 29.06.2021 22:10




Mathematics, 29.06.2021 22:10


Mathematics, 29.06.2021 22:10



Health, 29.06.2021 22:10

Mathematics, 29.06.2021 22:10

Mathematics, 29.06.2021 22:10



Mathematics, 29.06.2021 22:10

Mathematics, 29.06.2021 22:10

Mathematics, 29.06.2021 22:10

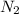 is 64.9 J/Kmol
is 64.9 J/Kmol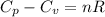
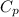 = heat capacity at constant pressure
= heat capacity at constant pressure 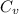 = heat capacity at constant volume
= heat capacity at constant volume