
Chemistry, 14.02.2020 18:09 natebarr17
A comic book villain is holding you at gun point and is making you drink a sample of acid. She gives you a beaker with 100ml of a strong acid with pH=5. She also gives you a beaker of a strong base with a pH=10. You can add as much of the strong base to the strong acid as you want, and you must then drink the solution. You'd be best off trying to make the solution neutral before drinking it. How much of the base should you add?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3


Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

You know the right answer?
A comic book villain is holding you at gun point and is making you drink a sample of acid. She gives...
Questions



Chemistry, 24.08.2019 05:00

Advanced Placement (AP), 24.08.2019 05:00

Biology, 24.08.2019 05:00

Geography, 24.08.2019 05:00

History, 24.08.2019 05:00

History, 24.08.2019 05:00




Mathematics, 24.08.2019 05:00

Advanced Placement (AP), 24.08.2019 05:00

Biology, 24.08.2019 05:00

Mathematics, 24.08.2019 05:00

History, 24.08.2019 05:00




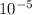 = [H⁺]1x10⁻⁵ M * 0.100 L = 1x10⁻⁶ moles H⁺
= [H⁺]1x10⁻⁵ M * 0.100 L = 1x10⁻⁶ moles H⁺


