
Chemistry, 14.02.2020 19:16 lindsaypre0644
What is the maximum amount of silver (in grams) that can be plated out of 4.6 LL of an AgNO3AgNO3 solution containing 3.3 %% AgAg by mass? Assume that the density of the solution is 1.01 g/mLg/mL.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
What is the maximum amount of silver (in grams) that can be plated out of 4.6 LL of an AgNO3AgNO3 so...
Questions


History, 17.12.2021 03:10






Biology, 17.12.2021 03:10





Social Studies, 17.12.2021 03:10


Computers and Technology, 17.12.2021 03:10

Computers and Technology, 17.12.2021 03:10


Medicine, 17.12.2021 03:10


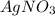 contains 3.3% Ag
contains 3.3% Ag

