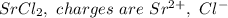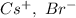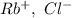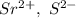Chemistry, 14.02.2020 19:38 alicemareus
The charges and sizes of the ions in an ionic compound affect the strength of the electrostatic attraction holding that compound together. Based on ion charges and relative ion sizes, rank these ionic compounds by their expected melting points from highest to lowest
a. SrCl2 ,b. CsBr ,c. RbCl ,d. SrS

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3


Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
The charges and sizes of the ions in an ionic compound affect the strength of the electrostatic attr...
Questions






Physics, 16.01.2020 02:31

Computers and Technology, 16.01.2020 02:31

Mathematics, 16.01.2020 02:31

Mathematics, 16.01.2020 02:31


Social Studies, 16.01.2020 02:31



Engineering, 16.01.2020 02:31


Computers and Technology, 16.01.2020 02:31




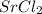 > RbCl > CsBr
> RbCl > CsBr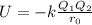
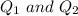 are charges and
are charges and 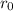 is the size.
is the size.