
Chemistry, 14.02.2020 23:13 uticabadgirl
Calculate the concentration of an aqueous solution of Ca(OH)2Ca(OH)2 that has a pHpH of 12.11. Express your answer using two significant figures.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
Calculate the concentration of an aqueous solution of Ca(OH)2Ca(OH)2 that has a pHpH of 12.11. Expre...
Questions



Health, 24.06.2019 11:40

History, 24.06.2019 11:40




English, 24.06.2019 11:40

English, 24.06.2019 11:40




Chemistry, 24.06.2019 11:40


Geography, 24.06.2019 11:40


Mathematics, 24.06.2019 11:40



 is
is 
![pH=-\log [H^+]](/tpl/images/0511/9847/37e81.png)
![pOH=-\log [OH^-]](/tpl/images/0511/9847/1fac1.png)


![1.89=-\log [OH^-]](/tpl/images/0511/9847/85b6a.png)
![[OH^-]=0.0129](/tpl/images/0511/9847/e7d0e.png)
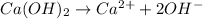
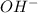 are produced by = 1 mole of
are produced by = 1 mole of 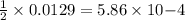 moles of
moles of 

